How We Help
MEDICAL AFFAIRS CONSULTING
Framework’s Medical Affairs (MA) is led by Stacey Benefiel, Pharm. D. Stacey’s over 25 years in industry, coupled with her team of renowned subject matter experts (SMEs), can partner, assist and guide Phase 2-4 bioscience companies that seek, require and value SMEs who are industry veterans, clinicians and scientists.
Bioscience companies can leverage and benefit from Framework’s MA practice to:
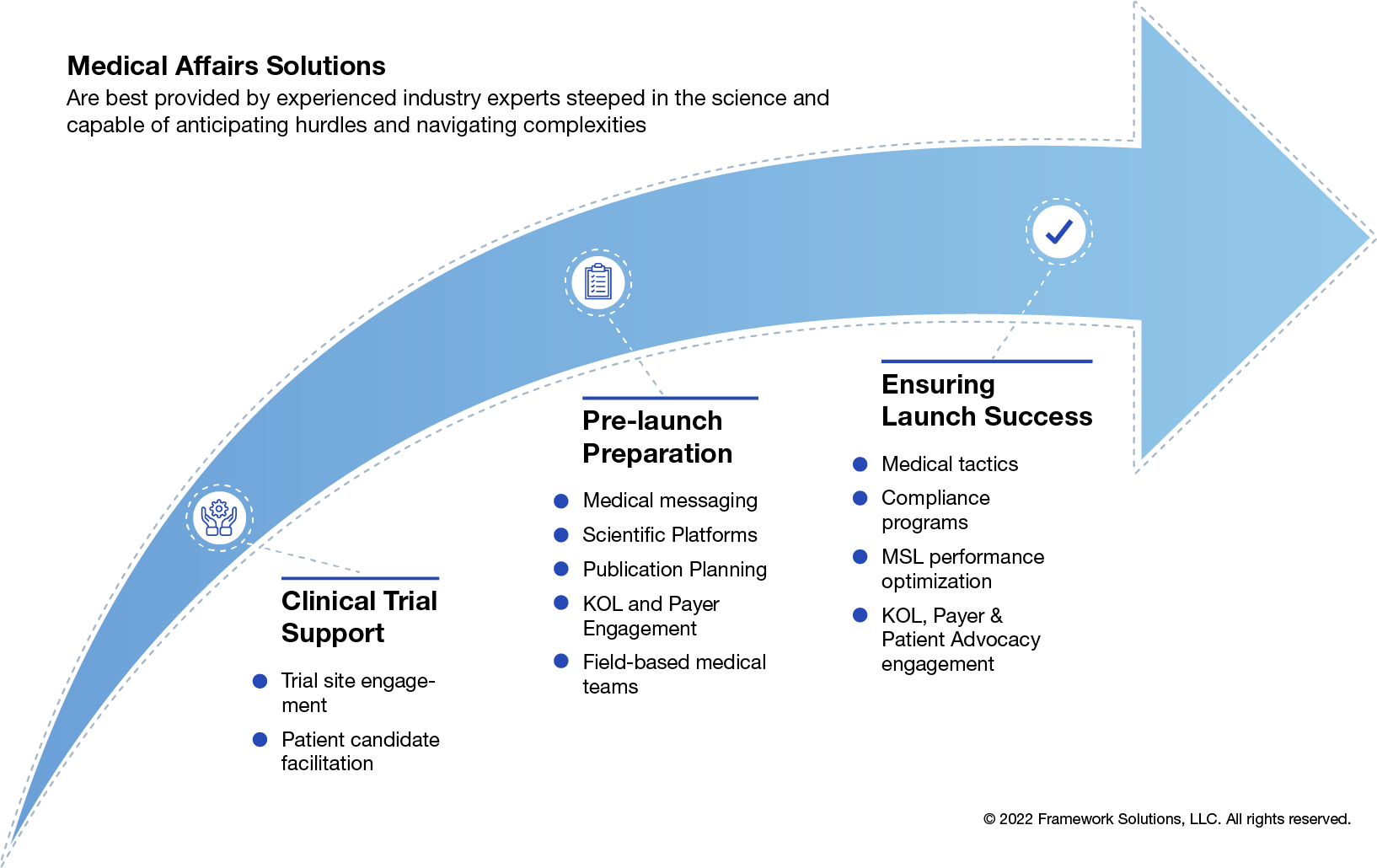
Frameworks MA team provides end-to-end (Phase 2-4) Clinical Development and complimentary Commercialization support as described in the diagram below:
Let’s connect.
"*" indicates required fields
Transformation through Collaboration




