Data Reports
Establishing a Content Management Process as a Late Stage Company
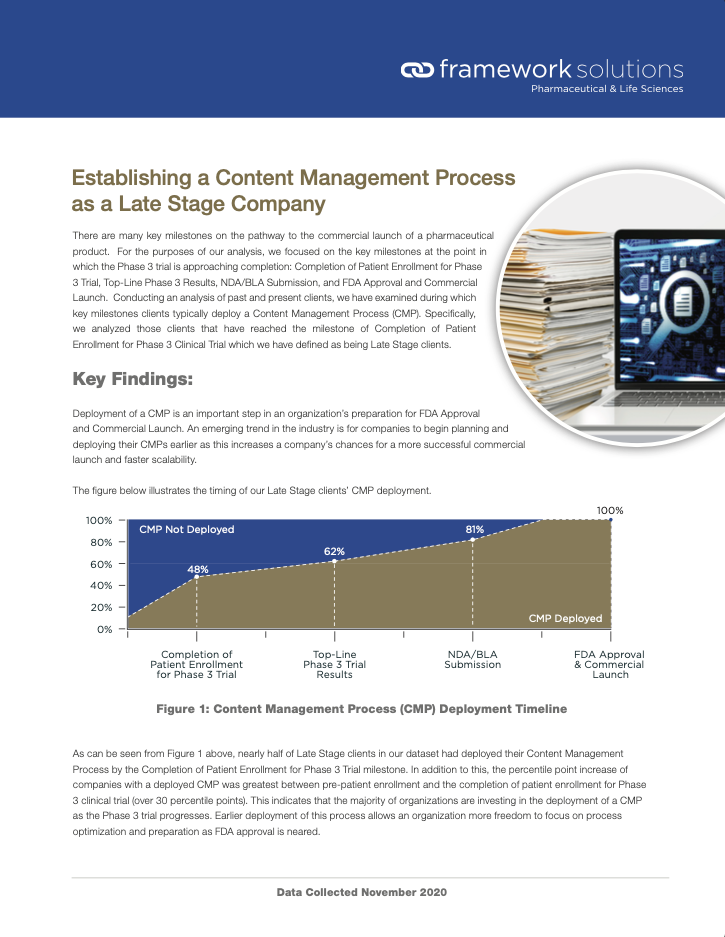
There are many key milestones on the pathway to the commercial launch of a pharmaceutical product, including the establishment of a Content Management Process (CMP). This summary provides learnings from our analysis of past and present clients of when CMP’s are typically deployed. More detailed information can be found within the White Paper:

Reference Management Process (RMP)
The creation of a robust Reference Management Process (RMP) can benefit your Content Management Process if executed consistently. This Data Report summarizes our Key Findings from a recent survey we performed on the impacts of a Reference Management Process. More detailed information on creating an RMP can be found within the White Paper:

Impacts of COVID-19 on Content Review and Approval Survey
Interested in learning how COVID-19 impacted Content Review and Approval in 2020, read this Summary Report to learn more about how 2020 impacted the shift from Print to Digital Content.
Transformation through Collaboration






