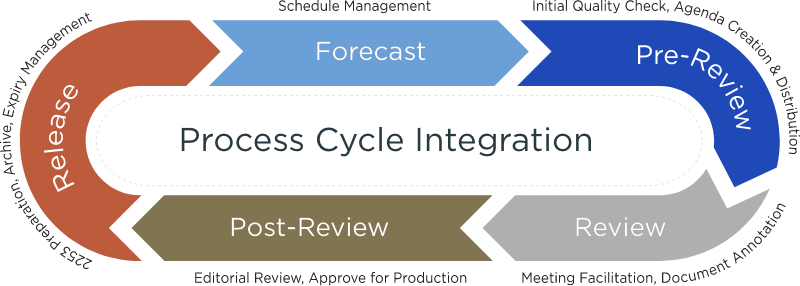
How We Help
We partner with agencies, project owners, and reviewers to ensure compliance, identify bottlenecks or process gaps and maintain an efficient, expeditious, and compliant process.
Our highly trained Coordinators are uniquely qualified to fill this role. Each has in-depth knowledge of all aspects of content review. They are well versed in FDA regulations, process optimization and SOP management. In addition, they are experts in all leading Content Management System platforms allowing for quick integration into your process.
Our Coordinator Services Team has facilitated the review of over 700,000 assets for many of the leading companies in the industry
Services include:
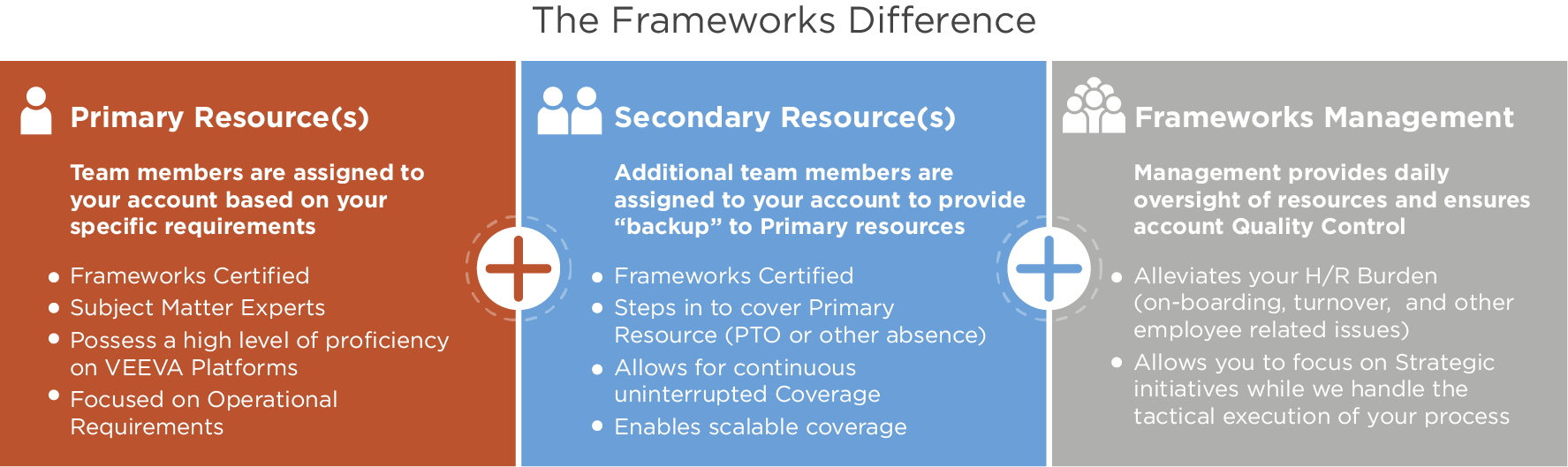
Benefits
Let’s connect.
"*" indicates required fields
Transformation through Collaboration